The Challenge
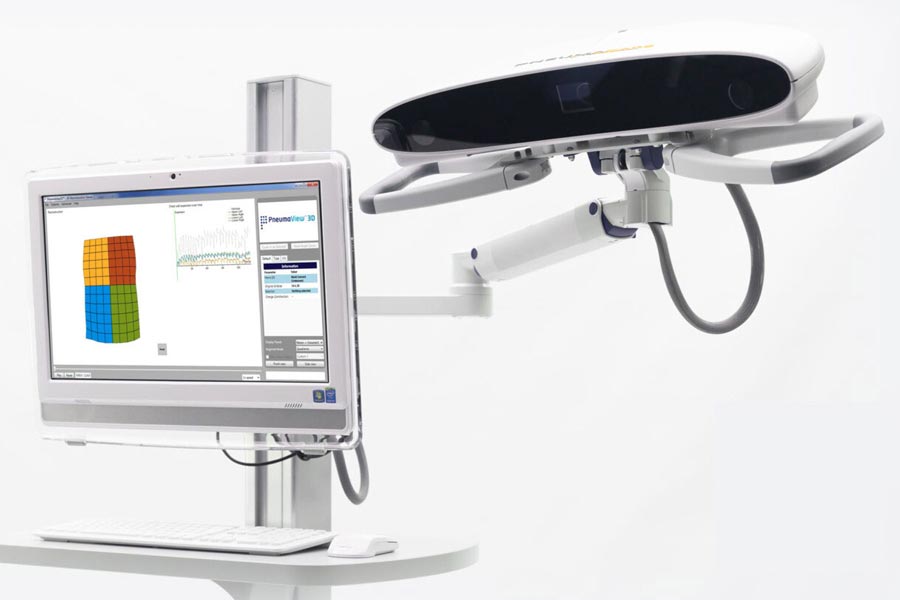
Our client PneumaCare, a University of Cambridge spin-out, had developed non-contact technology for the measurement of lung function, which did not require the patient to make forced expirations.
Their highly innovative approach used structured-light analysis to assess 3D movement of the chest.
PneumaCare asked Plextek to take their early prototype system through to manufacture, including regulatory approvals.
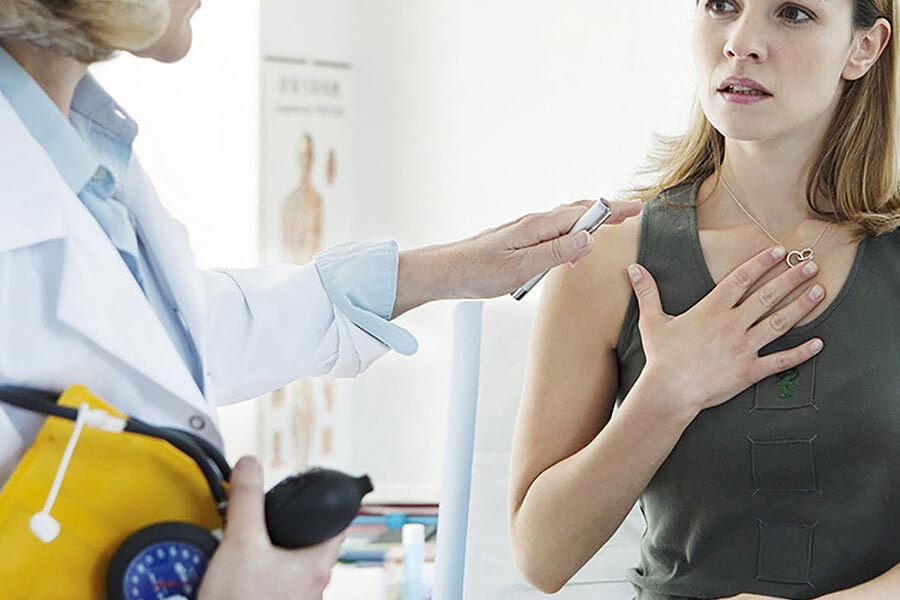
The Approach
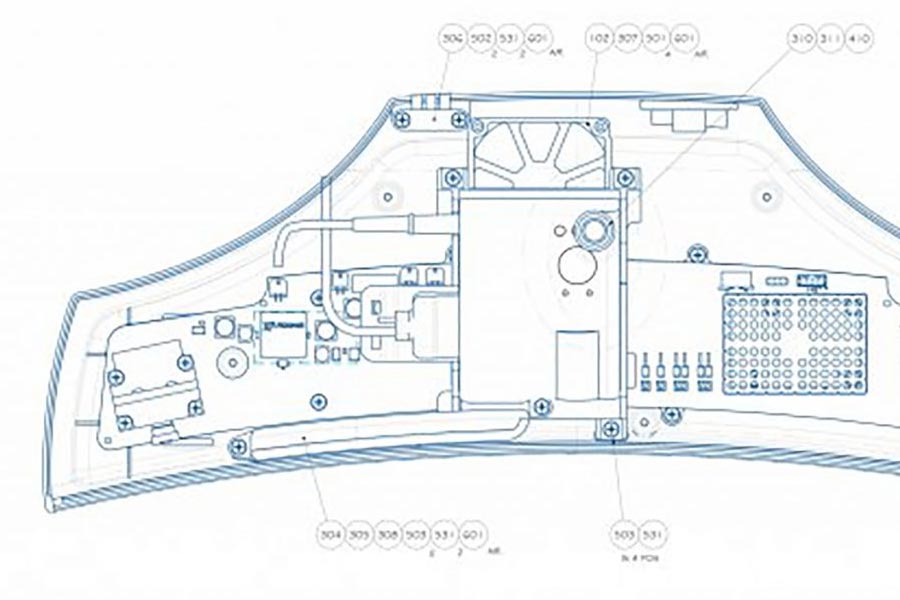
We realised that development of a commercial system would involve the solution of numerous technical issues, including stereoscopic camera alignment, effect of lighting conditions, reliability and calibration.
We undertook an initial technology feasibility study, conducted an IP search and a manufacturing assessment, and recommended the most cost-effective development approach.
We assembled a multi-disciplinary team experienced in medical device development, and worked closely with PneumaCare’s founders to realise their ambition.
The Approach
We realised that development of a commercial system would involve the solution of numerous technical issues, including stereoscopic camera alignment, effect of lighting conditions, reliability and calibration.
We undertook an initial technology feasibility study, conducted an IP search and a manufacturing assessment, and recommended the most cost-effective development approach.
We assembled a multi-disciplinary team experienced in medical device development, and worked closely with PneumaCare’s founders to realise their ambition.

The Outcome
Since launching this product, PneumaCare has won numerous medical device innovation prizes and received praise from leading Respiratory and Paediatric specialists.
The Thora-3DI™ device achieved CE mark approval in Europe in 2012, and is being used in hospitals in the UK, France, Italy, Denmark, Sweden, Middle East, Hong Kong, China and Malaysia.
Following the first FDA 510(k) clearance granted in March 2016, PneumaCare is now working with its strategic partners to make the device available in the US and in other markets.